Welcome AAO 2020 Virtual Attendees
BioTissue provides premium regenerative therapies for premium outcomes in ocular surface disease. BioTissue’s proprietary cryopreserved amniotic membrane, used in both the PROKERA® and AmnioGraft® family of products, is the only amniotic membrane FDA-designated as anti-inflammatory, anti-scarring, and anti-angiogenic.
Shaping the Future of Regenerative Medicine
Shaping the Future of Regenerative Medicine
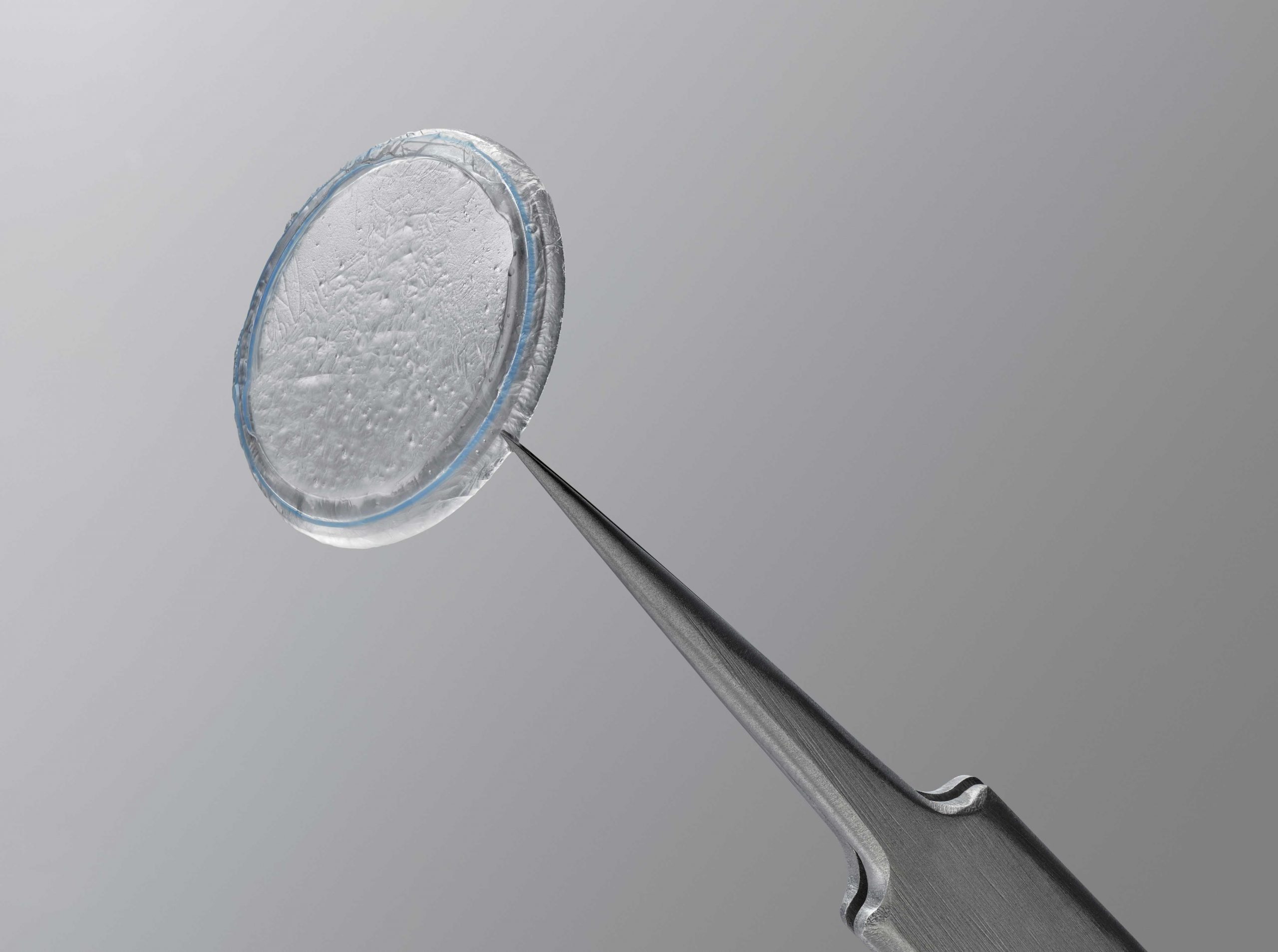
PROKERA is a biologic corneal bandage device used by eye doctors around the world to treat ocular conditions such as keratitis, moderate to severe dry eye disease, and many other ocular surface diseases.

AmnioGraft is a biologic ocular transplantation graft used by eye surgeons around the world as an adjunct therapy to treat ocular surface indications such as corneal ulcers, pterygium, mechanical dry eye (also known as conjunctivochalasis), excision of tumors, chemical burns, and Stevens-Johnson Syndrome.

AmnioGuard is an umbilical cord graft used by ophthalmic surgeons for a number of indications including to cover the tube shunt of a glaucoma drainage device.
Promote Regenerative Healing, Backed by Research
Our extensive research allows us to preserve and deliver the most functional allograft for your needs.
500K
TRANSPLANTS
360
CLINICAL PUBLICATIONS
30Y
RESEARCH & DEVELOPMENT
Find Your Wow Moment
We documented the positive impact our therapies have on patients all over the nation. Discover what PROKERA and AmnioGraft can do for your clinical outcomes, patient satisfaction and practice growth. Get ready to be wowed!
Discover Mechanical Dry Eye (MDE)
Discover what leading eye care specialists are saying about Mechanical Dry Eye (also known as Conjuctivochalasis) and their experience using AmnioGraft with Reservoir Restoration procedure for better patient outcomes.
Downloadable Resources
For AAO 2020 Virtual Attendees
- PROKERA Information Brochure
- AmnioGraft and AmnioGuard Information Brochure
- PROKERA Comparison Sales Aid
- PROKERA Family Flash Cards
- Redefining the Standard of Care: A Consensus Guideline
Physician Portal

