Partnerships
Why Partner with BioTissue
- Pioneer in amniotic tissue space
- Proven scientific data to support multiple platforms
- Robust clinical pipeline with multiple assets in late-stage FDA/BLA approval process
- Regulatory approvals, clinical data, and capital required create significant barriers to entry for competitors to enter amniotic tissue marketplace
- Strong Financial Profile with institutional investors (Essex Woodlands, Ballast Point)

BioTissue is the leader in innovative technologies using products derived from human amniotic membrane tissues. Since its inception in 1997, the Company has pioneered the clinical application of human placental tissues – more than 800,000 patients have been treated with BioTissue products and the Company’s groundbreaking scientific and clinical achievements have been documented in more than 390 peer-reviewed publications.
+40Y
National Institutes of Health (NIH) Funding
1M+
Human Clinical Applications
420
Peer-Reviewed Publications
1st
to establish Level 1 CPT Codes for Amniotic Membrane Transplantation
100+
Clinical Training Opportunities Each Year
BLA Vision
Long-term future growth
Our Focus
Unmet Patient Needs
We are focused on giving healthcare providers cost-effective solutions to successfully address previously unmet patient needs:
Ophthalmic and optometric indications

It is estimated that the total economic burden of eye disorders and vision loss in the U.S. is $139 billion.1
Advanced wounds
A conservative estimate of the cost of caring for these wounds exceeds $50 billion per year in the U.S.2-5
Musculoskeletal applications

In 2011, the total indirect and direct costs for musculoskeletal disorders was estimated to be $874 billion, or 5.7 % of U.S. Gross Domestic Product.6
Non-opioid alternative to managing pain
The total economic burden of prescription opioid misuse in the U.S. is $78.5 billion annually with an estimated 128 people dying each day after overdosing on opioids.7
The Right Healing. The Right Product.
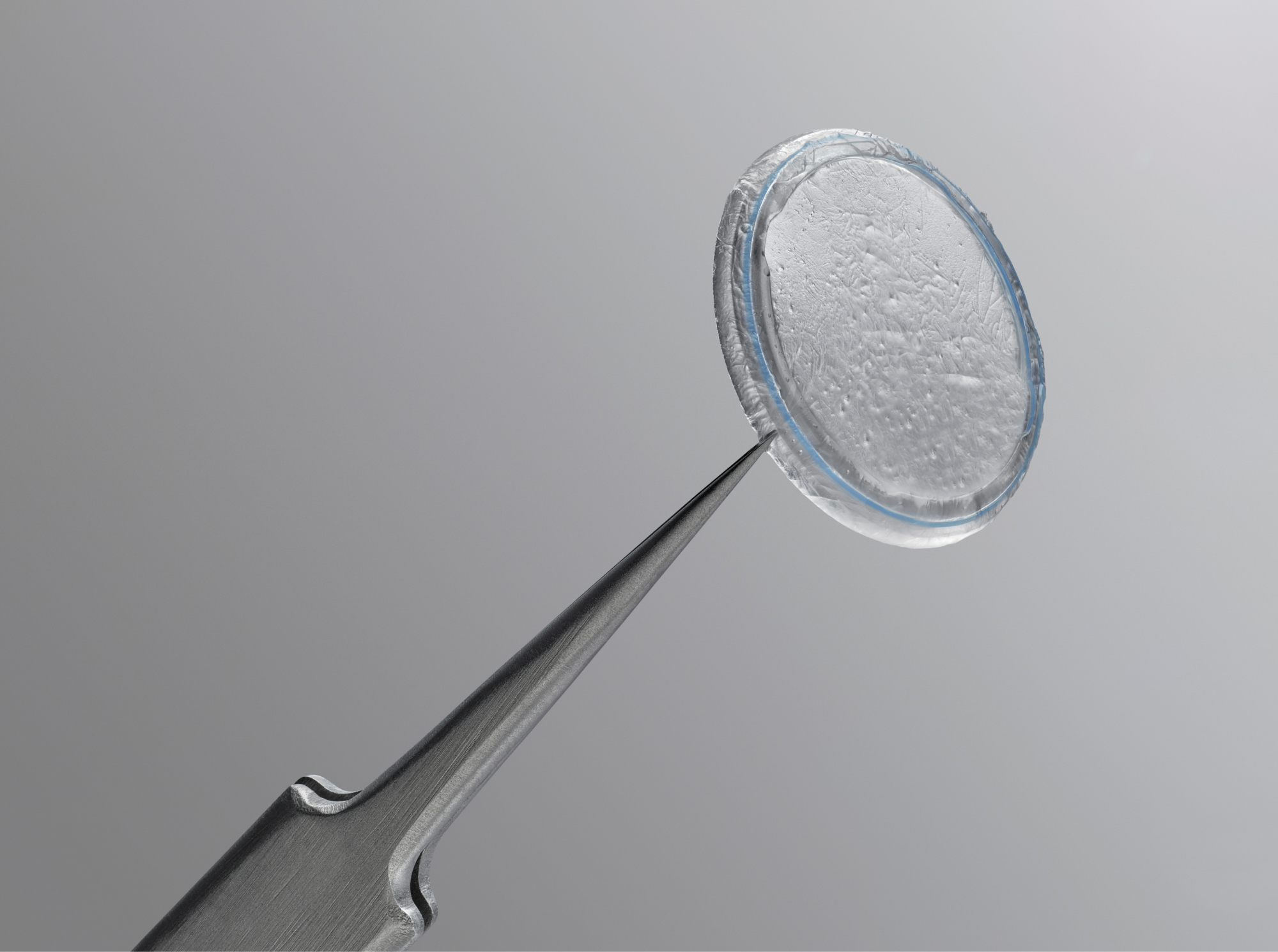
Prokera® Classic
Maintains orbital space with a symblephoron ring for cases where prevention of closure or adhesion is a concern.
Prokera Slim
Uses ComfortRing™ technology for a lower profile device that contours to the ocular surface to maintain comfort in treatment.

Prokera Plus
Maximizes the therapeutic benefit with a double layer of cryopreserved amniotic membrane tissue, for patients who need intensive treatment.
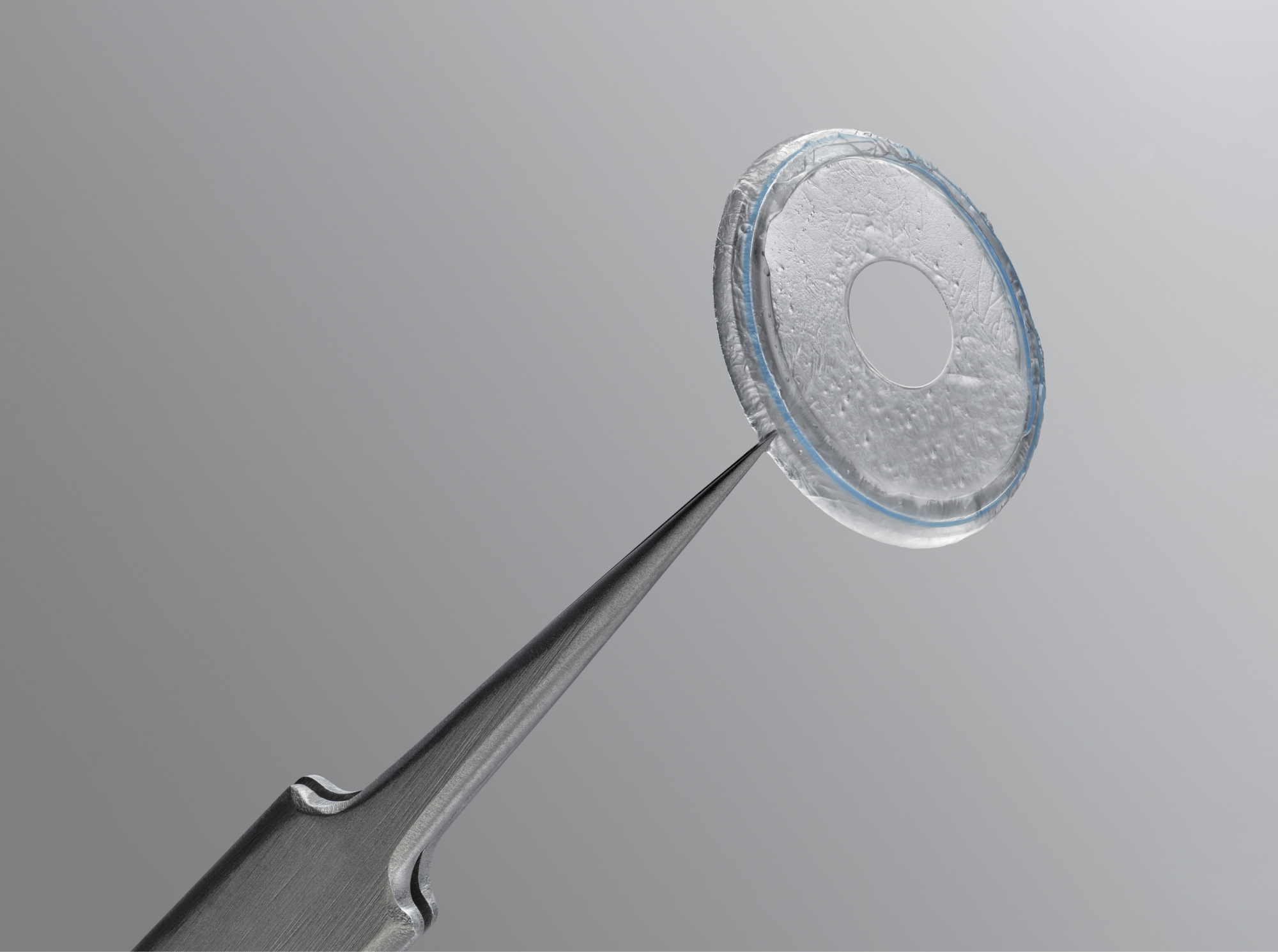
Prokera Clear
Provides patient with visual acuity during treatment with a 6mm ClearView™ aperture, which is crucial in monocular needs.
Rapid Recovery. Lasting Benefit.

AmnioGraft®, a cryopreserved amniotic membrane graft, supports accelerated post-op recovery and ensures superior patient outcomes when used in ocular surface reconstructive procedures.8-11
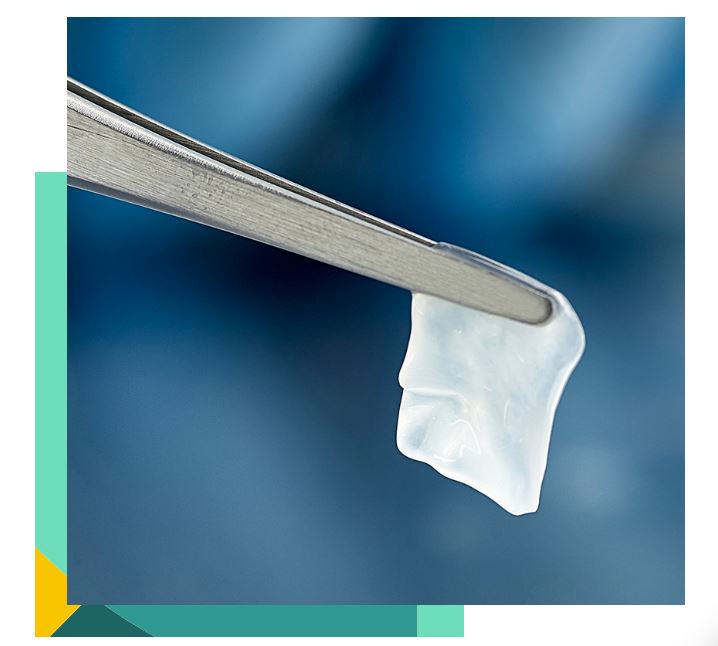
AmnioGuard® is an ultra-thick, cryopreserved amniotic membrane graft that suppresses inflammation, promotes healing, and provides durable tensile strength to avoid surgical challenges associated with reconstructive procedures.12-15
What Makes Our Ocular Products Different?
Offer your patients better healing with our cryopreserved amniotic membrane products, the ONLY amniotic membrane treatment:
Recognized by FDA for:
- Wound healing
- Reducing inflammation
- Minimizing corneal scarring16
- Inhibiting angiogenesis
Proven to be equivalent to fresh tissue17
- Mantains the biologic and structural integrity of the tissue.18
- Contains HC-HA/PTX3, a key protein found in fresh tissue.19
Meeting the highest quality standards20
- An AATB accredited tissue bank
- The only ISO 13485 certified manufacturer of corneal inserts incorporating amniotic membrane for ophthalmology
- Over 300,000 successful transplants
Demonstrating 20 years of proven clinical performance
- More than 350 peer-reviewed publications to date
- Associated with corneal nerve regeneration, which play a key role in tear film stability and ocular surface health21
- May accelerate healing of the ocular surface, with sustained reduction of signs and symptoms of dry eye22
Current
Physician Education & Training
We provide educational support through the following programs:
- BioTissue 360 Visit Programs
- Ocular Surface Biologics Course (OSBC)
- Leadership Training Summits
- Pro Ed Webinar Series
- DocMatter Peer-to-Peer Online Resource
- Physician Portal One-Stop Clinical Resource
- Local Peer-to-Peer

Practice Growth Support
- Provide superior outcomes for happier & loyal patients
- Establishment of Level 1 CPT Codes
- Favorable practice & patient economics compared to other standards of care
- Reimbursement and coding support
- Continued payer engagement
- Industry support via AAO (both), AOA, ASCRS, & Others
- Full consultative support from your rep and HQ team
- Trackable Med (direct to to patient advertising)

Marketing Tool Kit
The BioTissue Physician Marketing Toolkit provides physicians and their staff with simple tools for promoting our products on your print and digital marketing channels.

