Ocular
CAM360
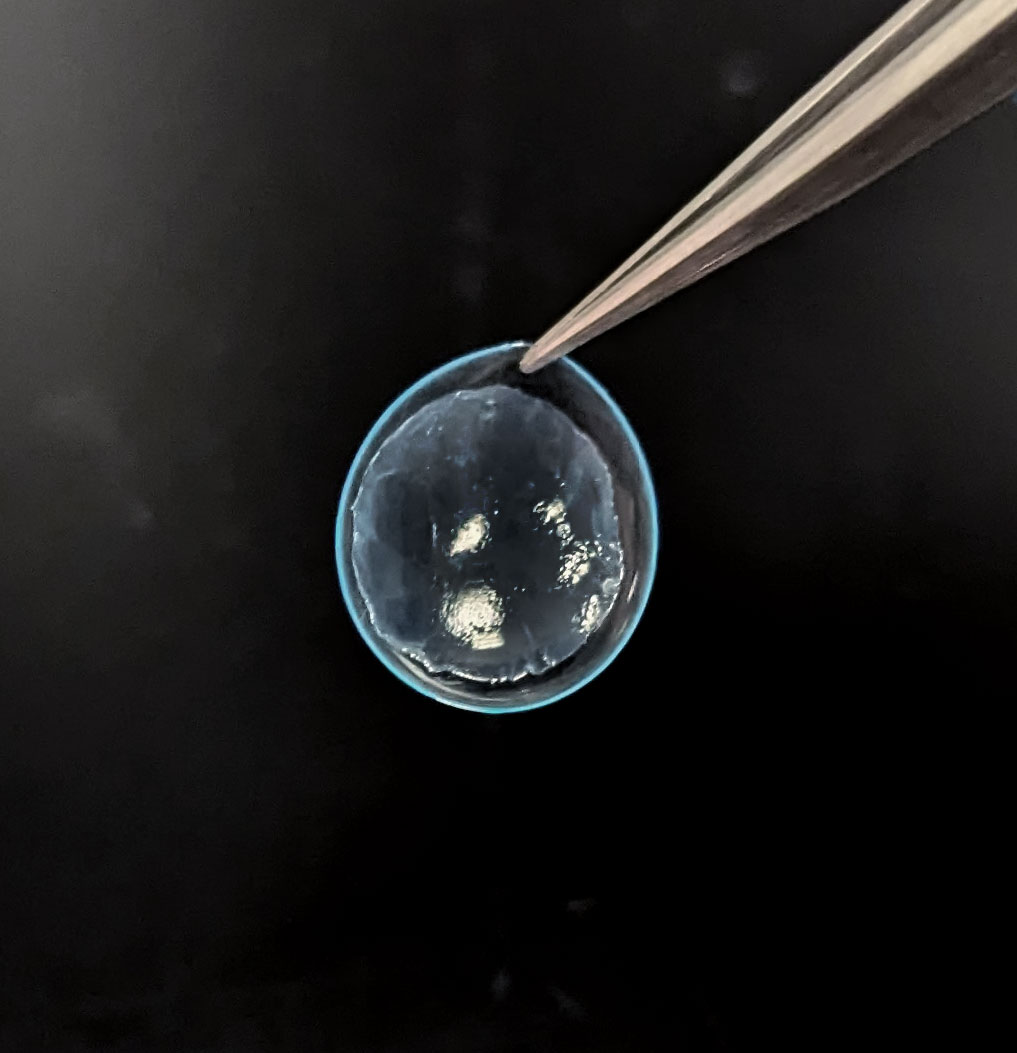
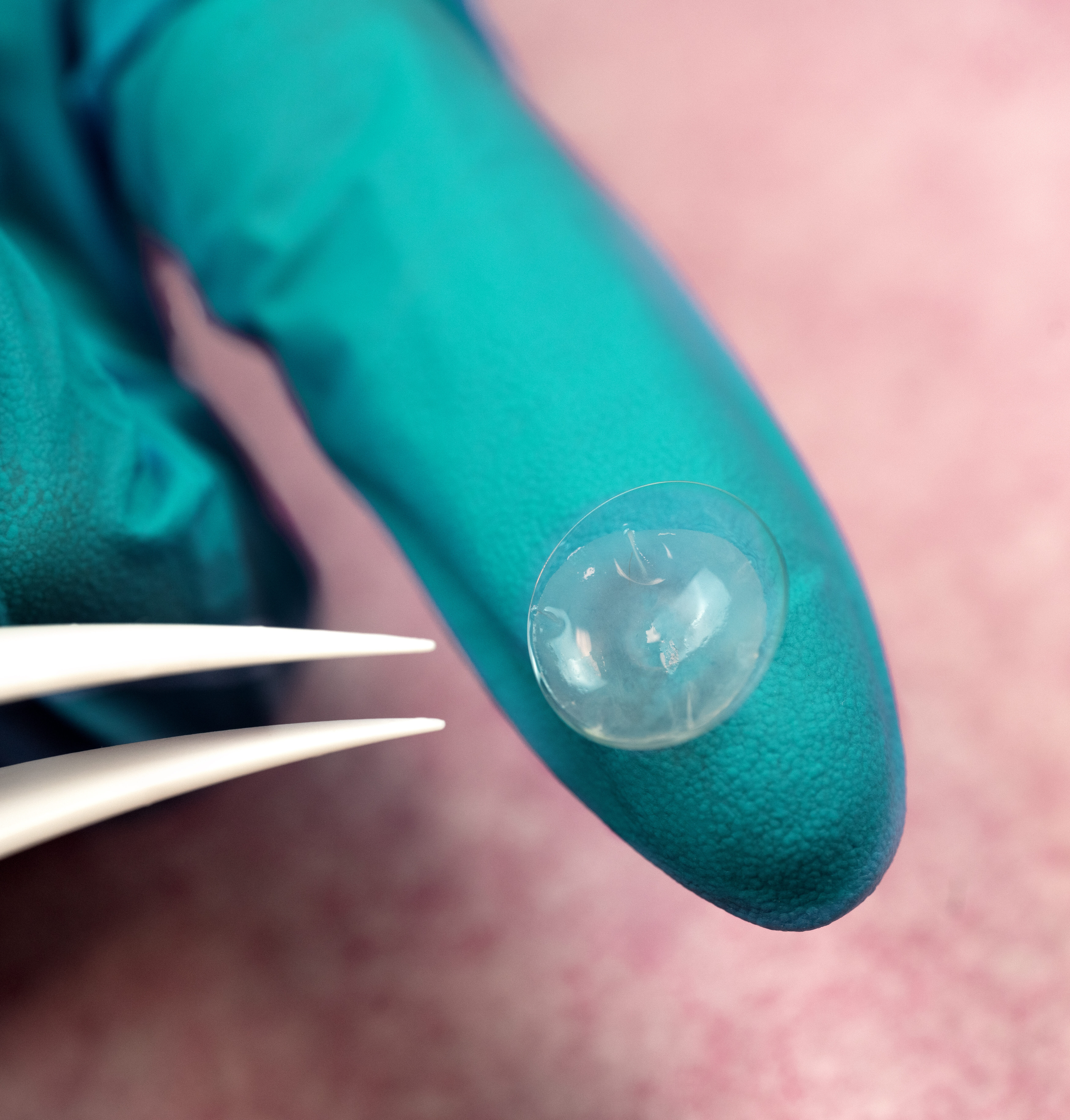
AmnioGraftTM
Developed to Optimize Comfort.
CAM360 AmnioGraft™ (CAM360 AG) is the first and only hydrated, cryopreserved amniotic membrane developed to optimize comfort that exerts anti-inflammatory, anti-scarring, anti-angiogenic properties for ocular use.1
Key Orchestrator
Preserving HC-HA/PTX3
HC-HA/PTX3 is a key matrix found within fresh and cryopreserved tissues that may be responsible for Amniotic Membrane’s (AM) therapeutic benefits.3
BioTissue’s proprietary CryoTek® and SteriTek® preservation methods are the only proven processing methods that retain the HC-HA/PTX3 found in fresh tissue.4 Our processing methods were specifically designed to optimize preservation while also providing efficiencies in handling, storage, and product application.
![]()
Sign up for our Physician Portal to access product info, clinical resources, and updates to support your practice!
Applications:
- Dry Eye Disease
- Superficial Punctate Keratitis (SPK)
- Ocular Surface Optimization
- Glaucoma-Medication Induced Dry Eye
- Neurotrophic Keratitis Stage 1
- Mild-to-Moderate Dry Eye Disease
CAM has been shown to:2,5-8
• Reduce signs and symptoms of Dry Eye Disease (DED)
• Support epithelial adhesion and differentiation
• Promote corneal nerve regeneration
Location & Temperature
-20°C → 25°C ( -4°F → 77°F)
Shelf Life
Use within the expiration date printed on product packaging (18 months from date of manufacture).
CAM360 AG Collagen Shield Application Video
Physician Resources:
Explore the resources below to support your practice and improve patient care.
For additional resources like clinical studies, product information, etc.
BioTissue Ocular Customer Support:
Call us Monday – Friday: 9:00 a.m. to 7:00 p.m. Eastern Standard Time.
Email: [email protected]
Call: (888) 296-8858
Fax: (305) 412-4429
For more information, visit our Ordering page.
Discover how CAM360 AG can Help Improve Patient Comfort

Testimonials
“Dry eye took over my life for the last few years without a solution I was satisfied with. With a simple treatment like CAM360 AG, I can return to my daily routine.”
– CAM360 AG Patient

Report any feedback or unexpected reactions by emailing [email protected].