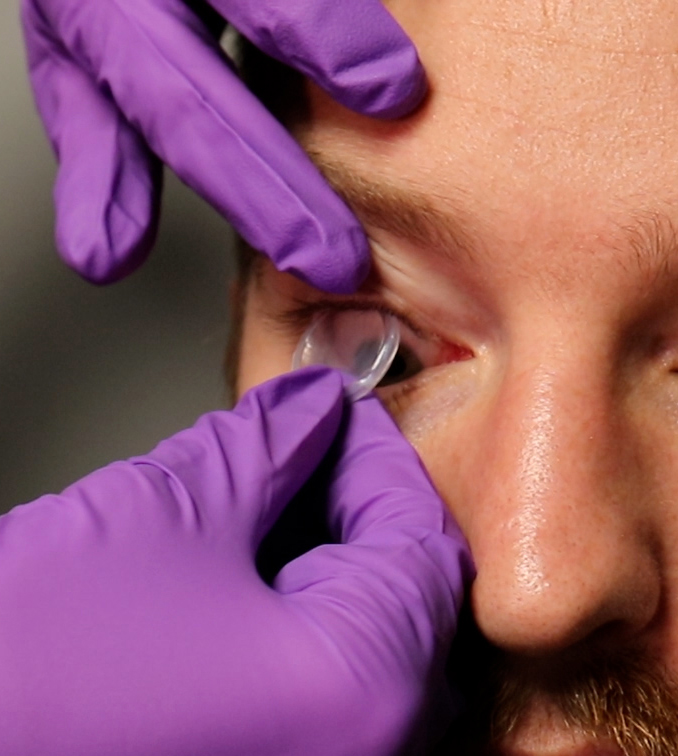
Ocular
Prokera
Healing Vision. Restoring Life.
Early intervention is critical in eye care. Prokera® corneal bandages are designed for treating damaged corneas by creating an environment for regenerative healing. Prokera helps expedite and empower the eye’s healing abilities for better patient outcomes.
Technology
Seeing a Difference
Our proprietary CryoTek® cryopreservation process maintains full biologic components including a key protein complex HC-HA/PTX3, and structural integrity equivalent to fresh tissue, which helps rapidly restore the cornea’s own healing capabilities.1,2
Prokera excels at restoring a normal and healthy epithelium while minimizing the risk of scar tissue formation. Heat dehydrated amniotic membrane grafts are processed in a way that destroys crucial biologic components, increasing the risks for repeated epithelial defects or erosions, chronic inflammation, scarring, vision loss, or pain.3,4
![]()
Sign up for our Physician Portal to access product info, clinical resources, and updates to support your practice!
The Right Healing. The Right Product.
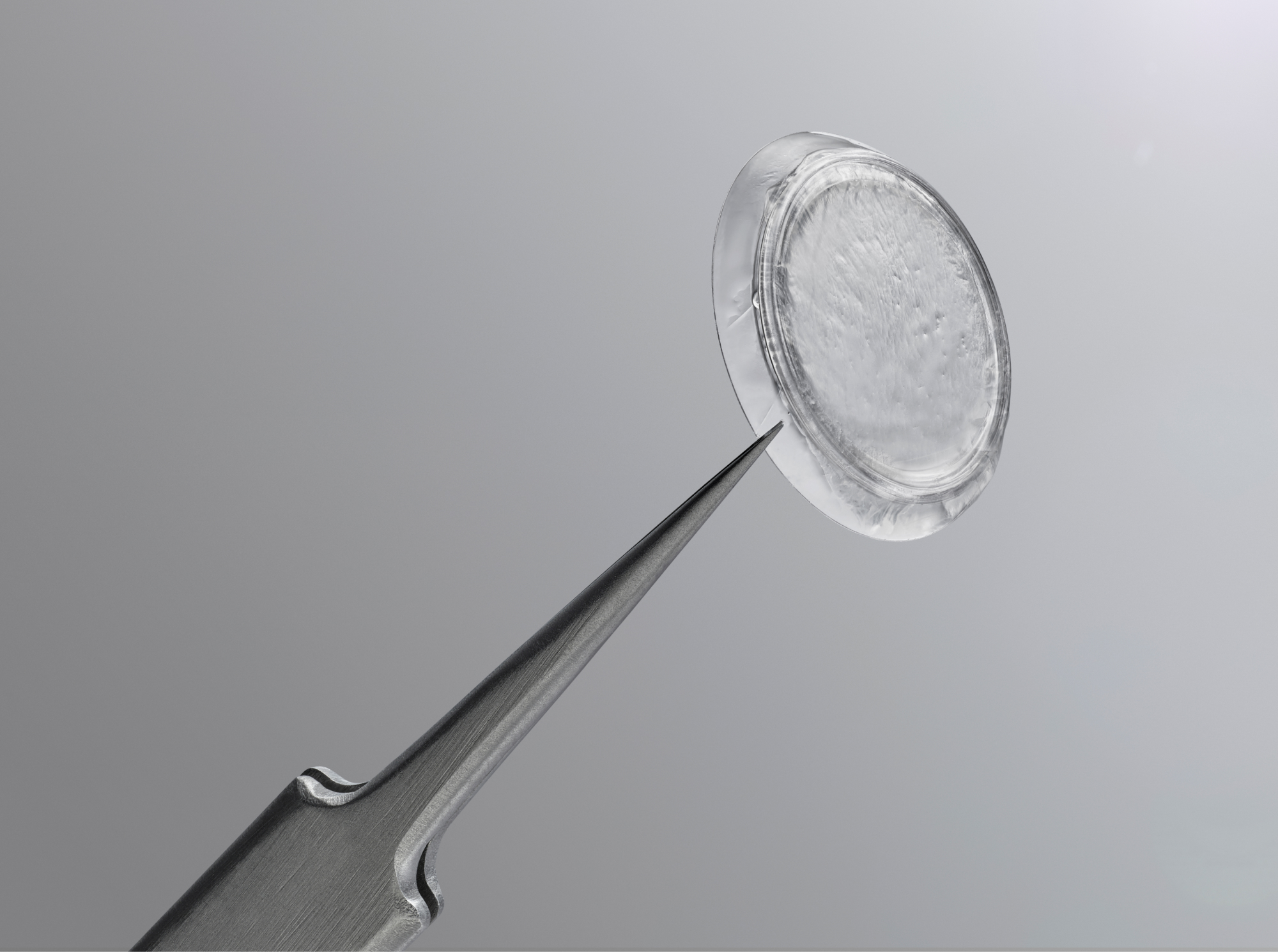
Prokera Classic
Maintains orbital space with a symblephoron ring for cases where prevention of closure or adhesion is a concern.
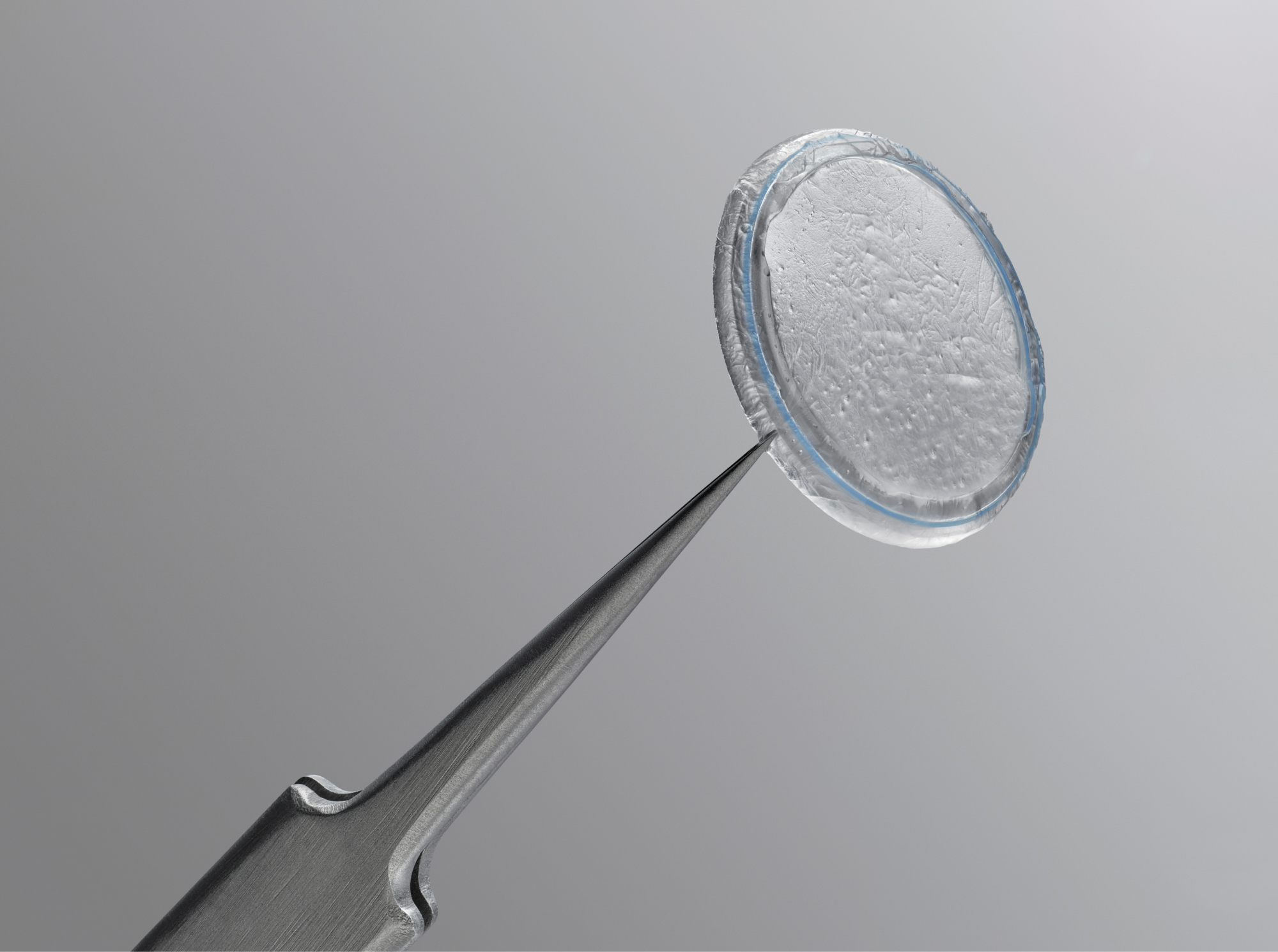
Prokera Slim
Uses ComfortRing™ technology for a lower profile device that contours to the ocular surface to maintain comfort in treatment.
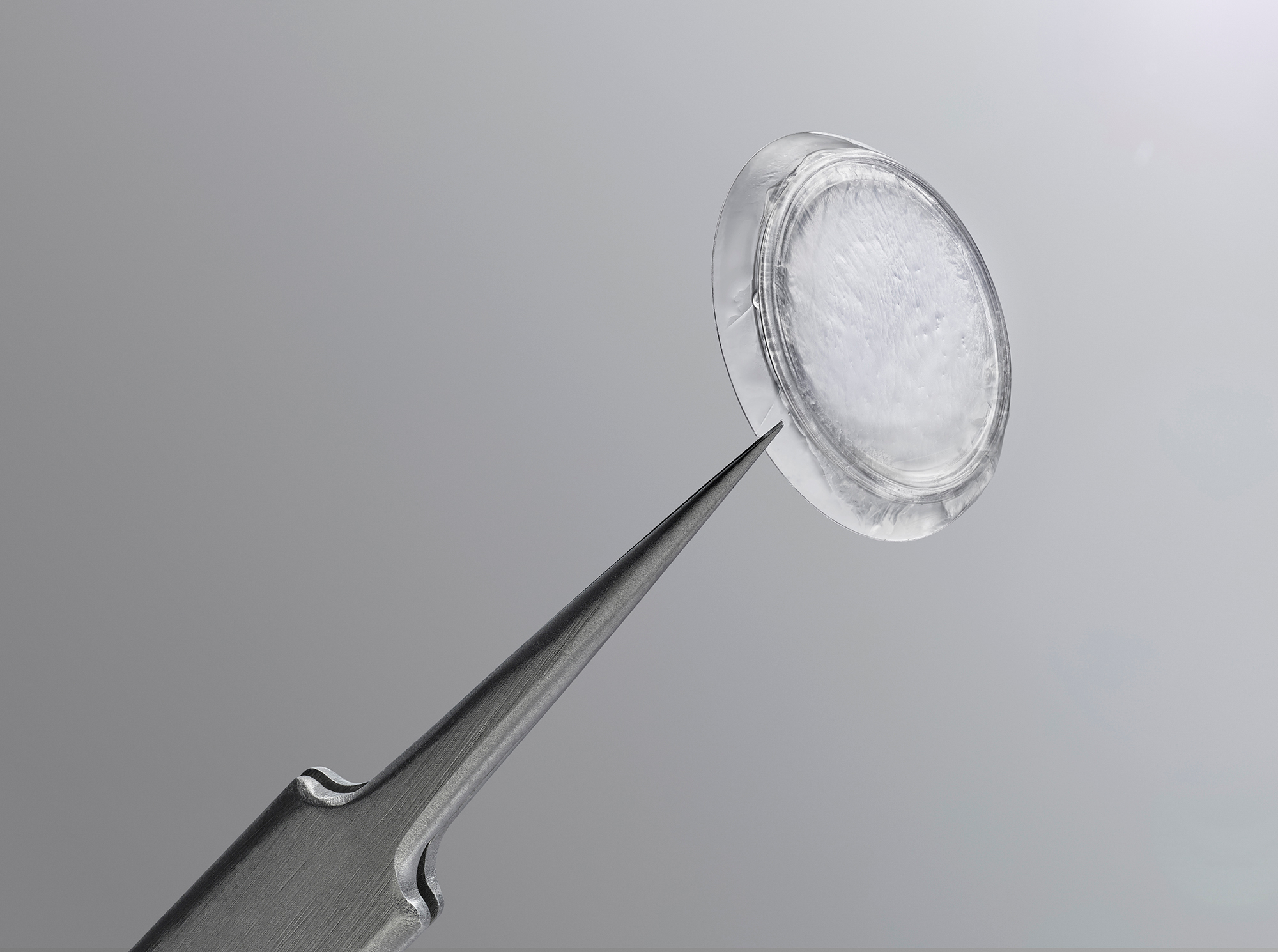
Prokera Plus
Maximizes the therapeutic benefit with a double layer of cryopreserved amniotic membrane tissue, for patients who need intensive treatment.
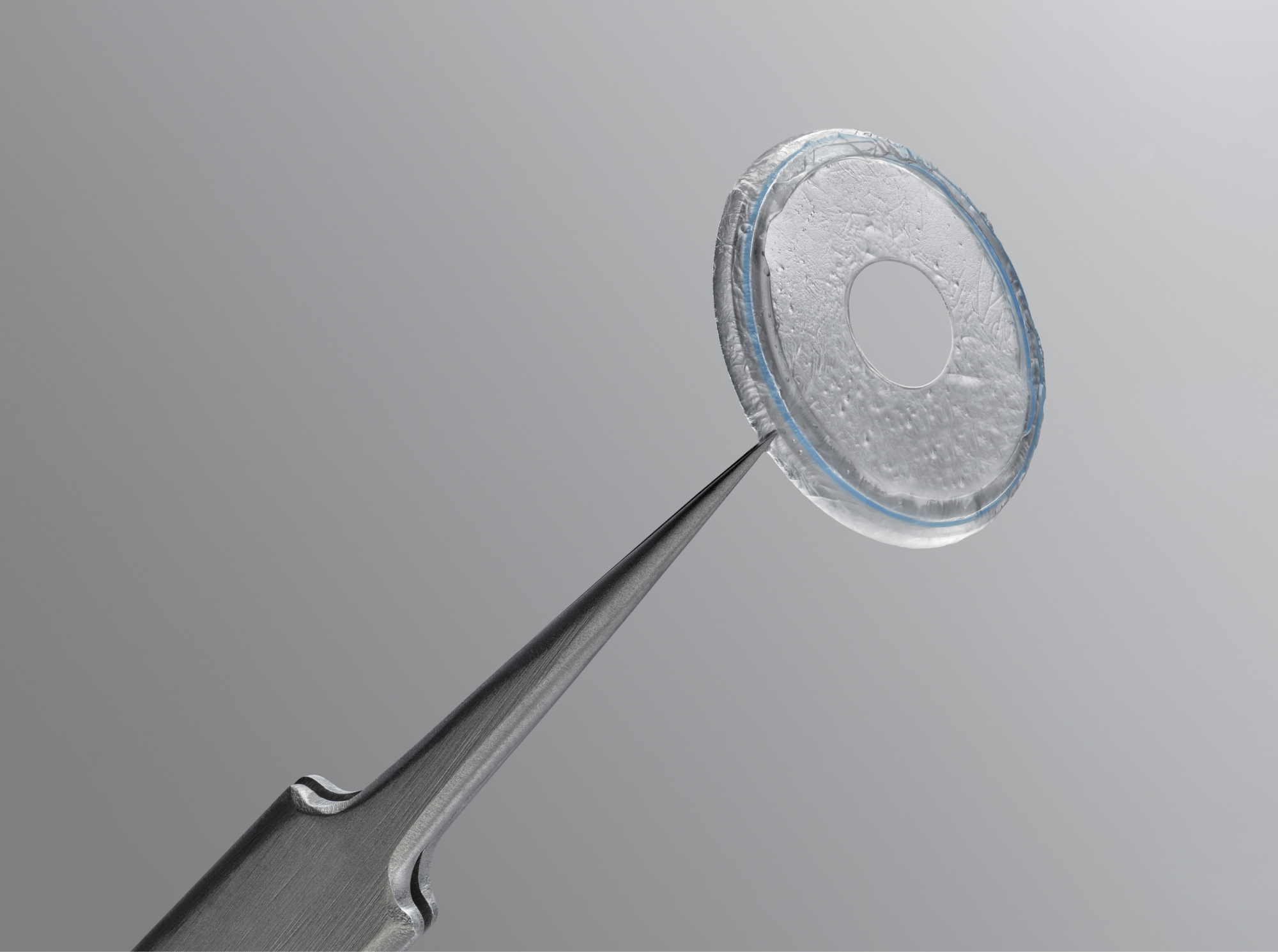
Prokera Clear
Provides patient with visual acuity during treatment with a 6mm ClearView™ aperture, which is crucial in monocular needs.
BioTissue products (Prokera, AmnioGraft, & AmnioGuard) are shipped in a temperature-controlled container and can be kept in the shipping container until the marked expiration date/time. Once removed from the shipping container, the products should be stored in a refrigerator or freezer according to the following guidelines:
Location & Temperature
Unopened insulated shipping container
-80°C → 4°C (-112°F → 39.2°F)
Example: ultra-low temperature freezer, standard freezer, or standard refrigerator
Shelf Life
Use within the expiration date printed on the outer shipping box.
Use within the expiration date printed on product packaging (2 years from date of manufacture).
Preparing Prokera for Use
- Remove Prokera from inner pouch
- Rinse Prokera thoroughly with sterile saline solution to prevent potential stinging from preservation media
- Patient may experience a temporary foreign body sensation upon insertion
- Download the Application Guide
Physician Resources:
Explore the resources below to support your practice and improve patient care.
For additional resources like clinical studies, product information, etc.
BioTissue Ocular Customer Support:
Call us Monday – Friday: 9:00 a.m. to 7:00 p.m. Eastern Standard Time.
Email: [email protected]
Call: (888) 296-8858
Fax: (305) 412-4429
For more information, visit our Ordering page.

Sharing Miracles
BioTissue Surgical obtains the tissue used in products through voluntary donation programs across the U.S. Before donation, donor mothers delivering through live, healthy C-section births are screened to ensure safety, based on requirements established by the U.S. Food & Drug Administration (FDA) and the American Association of Tissue Banks (AATB).
As Certified Tissue Bank Specialists, Sharing Miracles is in strict compliance with all Current Good Tissue Practices (CGTPs) as mandated by the U.S. Food and Drug Administration (FDA). Additionally, Sharing Miracles takes the extra step of certification by the American Association of Tissue Banks (AATB), adhering to their rigorous regulatory requirements for donated human tissue for transplantation.
Testimonials
“Before the Prokera I did not have crystal vision. Everything was blurry, had a hue to it, difficulty focusing on things. Now my vision is crystal clear, and I can see things I couldn’t see before. This is incredible. I’m just blown away.”
– Prokera Patient, Allentown, PA

Frequently Asked Questions
What is Prokera?
Prokera is made from amniotic membrane which has natural anti-inflammatory and anti-scarring properties. It is the only FDA cleared therapeutic device used by eye care practitioners to provide quick3 symptom relief and reduce inflammation associated with ocular surface disease. It helps restore your cornea and return your eye to a normal, healthy state.
What is amniotic membrane?
Amniotic membrane is part of the placenta and is the tissue closest to the baby throughout development in the womb. Amniotic membrane protects the baby from harm and has natural biologic properties which help the baby develop. The tissue has biological components that aid in ocular surface repair.
The amniotic membrane tissue in Prokera has natural therapeutic properties that help damaged eye surfaces heal. Eyes treated with Prokera have less scarring and less inflammation. The amniotic membrane in Prokera is thin and clear like the tissue on the surface of your eye and protects your eye’s damaged tissue while inserted.
Where does the amniotic membrane used in Prokera come from?
The tissue is donated by consenting healthy mothers after scheduled cesarean section (C-Section) births within the United States. Donor suitability is stringent and is determined through social, physical, and medical screening.
What does Prokera treat?
Prokera is used by eye doctors to treat eye diseases such as keratitis, corneal scars, chemical burns, corneal defects, partial limbal stem cell deficiency, and many other inflammatory ocular surface diseases.