Our Company
BioTissue is the leader in innovative technologies using products derived from human amniotic membrane tissues. Since its inception in 1997, the Company has pioneered the clinical application of human placental tissues – more than 900,000 patients have been treated with BioTissue products and the Company’s groundbreaking scientific and clinical achievements have been documented in more than 400 peer-reviewed publications.
Promote Regenerative Healing, Backed by Research
Our extensive research allows us to preserve and deliver the most functional allograft for your needs.
+40Y
Research & Development
1M+
Human Clinical Applications
420
Peer-Reviewed Publications
Mission
We empower healthcare professionals to deliver optimal patient healing outcomes, setting the standards that define next-generation care.

Vision
Realize the full potential of regenerative therapy.
Our Core Values:
- We CARE – Compassionate, Appreciative, Respectful and Empowered – for our donors, patients, providers, and our people.
- We Build Trust.
- We Take Ownership.
- We Deliver Excellence.
- We are ONE TEAM.


Our Foundation of Healing
The first reported clinical application of Amniotic Membrane (AM) was for skin transplantation in 1910; however, it wasn’t until 1995 that our Chief Technology Officer, Scheffer C.G. Tseng, MD, PhD and his colleague J.C. Kim reported the use of AM for ocular surface transplantation in a rabbit limbal stem cell deficiency model.1 They found that glycerin-preserved human AM promoted corneal recovery in rabbits that would sometimes fail with stem cell transplantation alone.
It appeared that the AM acted as a substrate to support the regeneration of corneal epithelium, just as good topsoil in the garden supports the growth of newly-planted seeds. This study suggested—for the first time—that inadequate wound healing lies in the lack of a supporting environment, and AM transplantation can serve as a surrogate niche environment to facilitate regenerative wound healing.
Our Breakthrough Discovery
The therapeutic effect of AM is believed to originate from its innate wound healing, anti-scarring and, most importantly, anti-inflammatory properties. In 2001, when cryopreserved AM received approval for ocular surface reconstruction through Request for Designation from the Food and Drug Administration (FDA), it was recognized to promote healing through anti-inflammatory, anti-scarring, and antiangiogenic effects.
Since that time, we have pondered the following question: What is in birth tissue that is responsible for these amazing results? One may naturally assume that AM’s complex actions are likely to be based on a symphony of biological molecules, but our cumulative research over the last decade suggests otherwise. Between 2002 and 2013, our research has focused on isolating the key molecules in birth tissue that contributes to its healing properties. We successfully identified and purified HC-HA/PTX3,2 a unique complex naturally present in AM and Umbilical Cord (UC) that orchestrates multiple anti-inflammatory, anti-scarring and anti-angiogenic effects and plays an important role in promoting regenerative wound healing as evidenced in the ocular segment.3-9
Preserving Mother Nature
We have developed a unique processing method (CryoTek®) to preserve HC-HA/PTX3 and growth factors/cytokines from the birth tissue while devitalizing living cells. In contrast, tissues processed via heat dehydration are structurally compromised and contain significantly less of the HC-HA/PTX3 complex.12,13
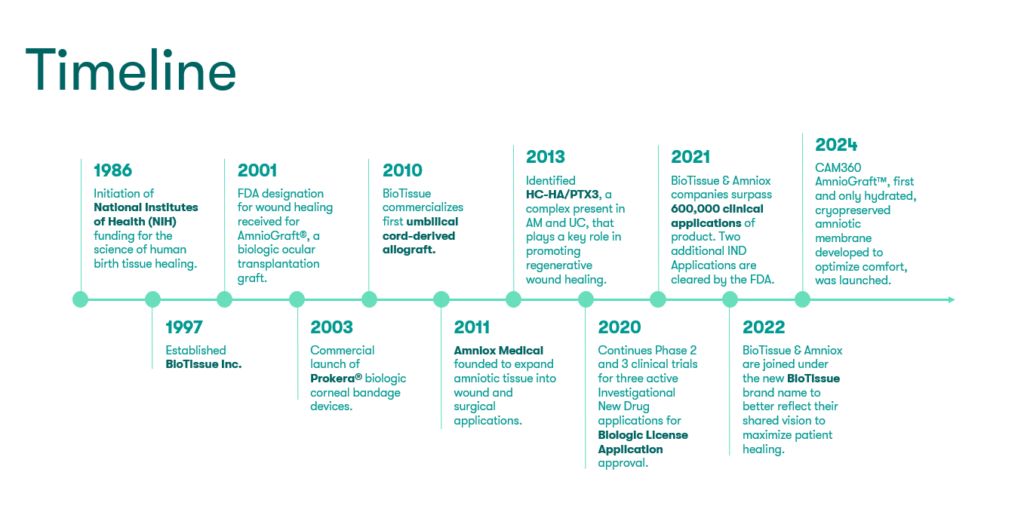
Our History
TissueTech was founded in 2001 as the parent entity for BioTissue Inc., which develops and markets regenerative therapies for treating the ocular surface. In 1997, BioTissue was the first company to introduce the use of cryopreserved amniotic membrane tissue for clinical application, by utilizing its proprietary CryoTek technology, proven to preserve the innate biological and structural properties of the amniotic membrane tissue.
In 2011, our company expanded from ocular space and its product offerings that, include Prokera®, AmnioGraft® and AmnioGuard® to additional market segments such as orthopedics,14-16 neuropathy,17 wound care,18-23 spine,24 urology25, and pain management26,27 using the same birth tissue products processed using our patented manufacturing technology. Our Neox® and Clarix® allografts are marketed as structural tissue products for homologous use as protective barriers and wound coverings. These products are manufactured and distributed in compliance with the regulatory requirements of 361 HCT/Ps that are regulated solely under section 361 of the Public Health Service Act and 21 CFR Part 1271.
Our Future Journey
In 2022, TissueTech has unveiled a corporate rebranding, including a name change and a new logo. BioTissue, Inc. and Amniox Medical, Inc. will now both be known under a single commercial, customer-facing entity, BioTissue, Inc. With three decades of continual advancements in regenerative medicine, BioTissue has been a clear leader in the ocular space. By adopting the BioTissue name across the entire organization, the company will also reflect that innovative heritage and promise in its surgical business, which was previously known as Amniox Medical.
BioTissue is pursuing FDA approval through submission of a Biologics License Applications (BLA) for some of our products as evidenced in a recently published Phase 2 study for complex diabetic foot ulcers.20 Our company will continue to provide sustainable health economic value, solve unmet clinical needs, and lead in technological innovation in seeking to deliver the promise of regenerative healing for our physicians and patients.
Company Recognition
2014: recognized as one of the 5000 Fastest Growing Incorporated Private Companies in the United States
2014: The Greater Miami Chamber of Commerce named its business unit BioTissue Minority-Owned Business of the Year.
2015: recipient of the prestigious Tibbetts Award from the U.S. Small Business Administration (SBA)
2017: received the award for Spine Technology Innovation in Biologics at the North American Spine Society annual meeting
2020: recognized on MD Tech Review’s annual listing of ten companies as “Top Companies in Wound Care Market”
One Company. One Purpose. One Vision.
Our company was founded on a commitment to patient healing, working alongside surgeons and other healthcare professionals who also share a passion for healing and changing patients’ lives. Learn about our pioneering company history and our legacy for a healthier future together.