March 9, 2016
Rejuvenating the Ocular Surface
published on March 09, 2016 by
Inflammation and regeneration share a close relationship in the human body. However, the pathological inflammatory process is an impediment to the body’s ability to regenerate. What my colleagues and I have learned through our NIH-funded research is that regeneration can take place only when inflammation is effectively controlled. Our discoveries have led to the development of amniotic membrane (AM) products for healing and rejuvenating the ocular surface. These include AmnioGuard® biologic glaucoma shunt tube graft; AmnioGraft® biologic ocular transplantation graft; and Prokera® biologic corneal bandage (BioTissue), and we’re working on applying our findings to a broader area in regenerative medicine.
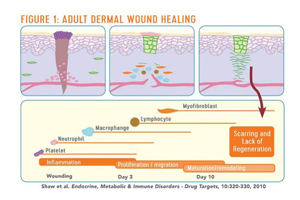
Our research shows that adult wound healing is remarkably different from fetal wound healing. Adult wound healing is carried out through sequential steps of cascade, starting from inflammation and progressing through a number of cell types. In the end, we see healing, but not total regeneration. Instead, we see scar tissue (Figure 1). Conversely, if a large tumor is removed mid-gestation with fetal surgery, the baby will be born with no scar.
Although stem cells have been seen as the key to regenerative medicine, they are only part of the picture. It’s well established that corneal epithelial stem cells reside in the limbus. We developed the conjunctival limbal autograft procedure1 as a way to transplant the stem cells and treat limbal stem cell deficiency, a condition in which the corneal epithelium is unable to repair and renew itself and is replaced by conjunctival epithelium.
Early on, we saw that the procedure was effective in some cases but not others, because simply transplanting stem cells to diseased tissue doesn’t lead to regeneration if inflammation lingers. Stem cells require a specific environment, known as a niche, that is free of inflammation in order to carry out their functions, and it appears AM can provide that niche environment.2 This concept is demonstrated in our paper published in 1997 showing that AM transplantation can be a substrate for conjunctival surface reconstruction.3 Our continuous research efforts since then have shown that fresh AM is not a structural scaffold; rather it is biological in action.
In further exploring exactly how AM is capable of fostering regeneration, we have spent a total of 12 years identifying a unique matrix component, HC-HA/PTX3, which is responsible for its anti-inflammatory, anti-scarring, and anti-angiogenic actions.4 We found out that AM is the only tissue other than the liver that can produce inter-α-inhibitor, which is an essential component that is needed for biosynthesis of this matrix.5 HC-HA/PTX3 can exert multiple functions to multiple cell types, and we believe it’s the key to modulating the stem cell niche and replicating the fetal wound healing process.6,7 It’s important to note that our findings pertain only to AM preserved with the CryoTek® cryopreservation method, which, unlike AM preserved by dehydration, maintains the quantity and activity of HC-HA/PTX3 in fresh AM.8 Based on our knowledge of the HC-HA complex, we now have the platform technology that can be applied to the development of novel therapeutics in ophthalmology and beyond.
To learn more about how cryopreserved amniotic tissue products can help your patients, download the “How Do You Define Optimal Corneal Healing” brochure.
Scheffer C. G. Tseng, MD, PhD is chief scientific officer at BioTissue, and medical director of the Ocular Surface Center, Ocular Surface Research and Education Foundation in Miami.
