New Medicare Local Coverage Policies for CTPs/Skin Substitutes
We've Got You Covered
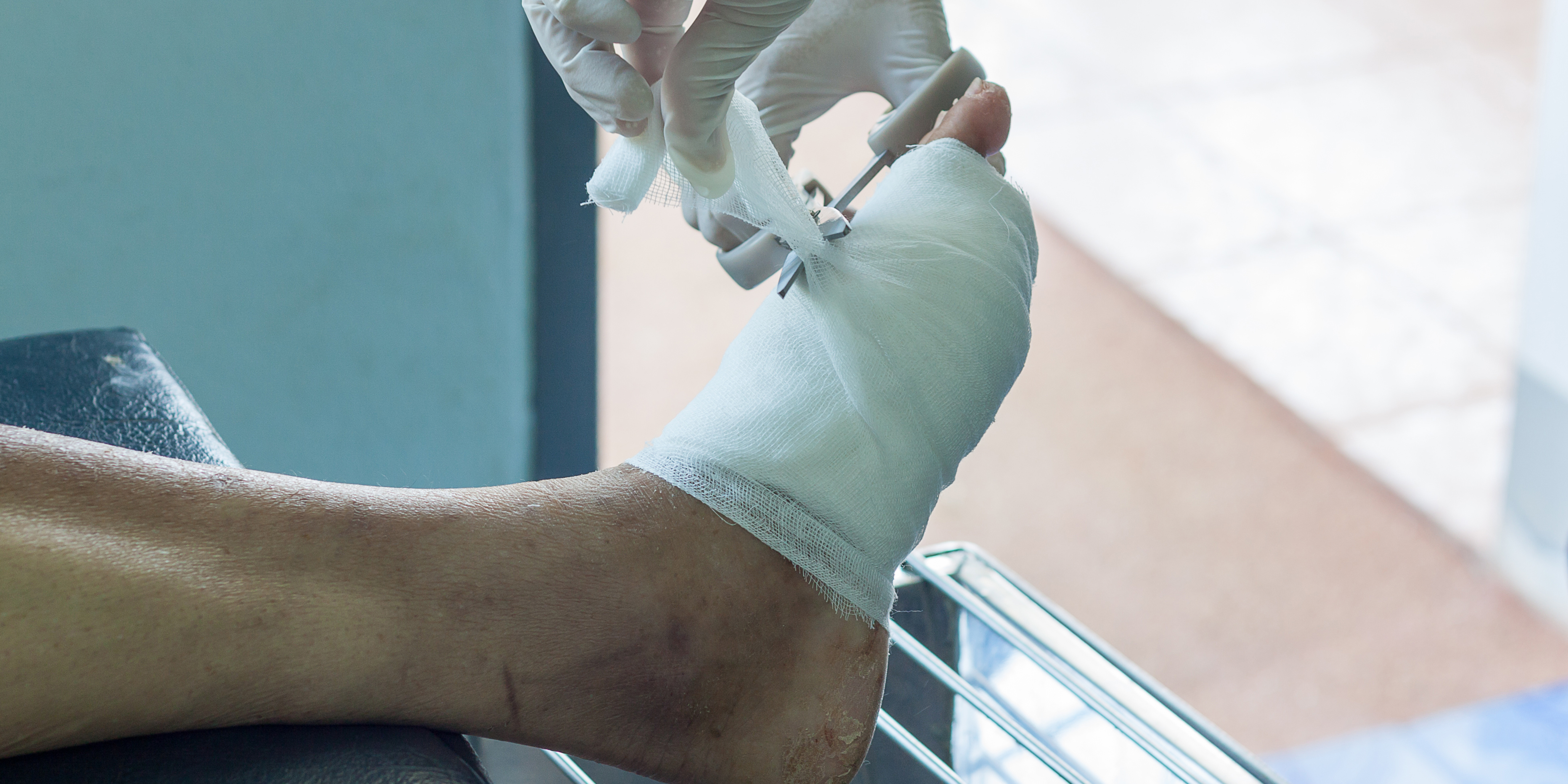
Clinical study results showed that 79% of patients with Wagner 3-4 achieve wound closure with an average of 1.24 applications.1
Are you aware of the current coverage policy change?
Hear from Tim Hunter, Vice President of Reimbursement & Government Affairs

Novitas: Arkansas, Colorado, Delaware, Louisiana, Maryland (Montgomery & Prince George’s counties), Mississippi, New Jersey, New Mexico, Oklahoma, Pennsylvania, Texas, Virginia (Arlington & Fairfax counties, Alexandria), Washington DC
See coverage policy LCD L35041 / LCA A54117
First Coast: Florida, Puerto Rico, & U.S. Virgin Islands
See coverage policy LCD L36377/ LCA A57680
CGS: Kentucky, Ohio
See coverage policy LCD L36690 / LCA A56696
Fewer Applications, Faster Healing.
If your practice is in one of the states affected by these changes, we’re here to help. Our coverage inclusion supports our vision of treating more patients, with higher healing rates with less applications in support of escalating healthcare costs in the patient population.

Discover BioTissue’s advanced wound care products:
Learn More How Neox Can Help You Navigate these Changes.
Fill out the details below and your local representative will contact you shortly.
Frequently Asked Questions
How does this coverage change impact your clinical practice?
- Neox the CTP which you are currently utilizing is covered, your choice in advanced wound care product will not be impacted.
- The LCD link provides products that are covered (Table 2) and non covered (Table 3)
What are the Neox® covered Q Codes?
- Q4148 Neox 1K, Neox RT and Clarix 1K per square cm
- Q4156 Neox 100, Clarix 100 per square cm
Do these changes impact the number of applications of advanced wound care products?
The number of CTP applications will be capped at 4 versus the current allowable of 9.
Do you have evidence that supports a lower number of applications to wound closure?
Our coverage inclusion supports our value based proposition of less applications with a vision of treating more patients, with higher healing rates with less applications in support of escalating healthcare costs in the patient population.
- Caputo WJ, Vaquero C, Monterosa A, Monterosa P, Johnson E, Beggs D, Fahoury GJ. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen. 2016 Sep;24(5):885-893. doi: 10.1111/wrr.12456. PMID: 27312890.
- Raphael A. A single-centre, retrospective study of cryopreserved umbilical cord/amniotic membrane tissue for the treatment of diabetic foot ulcers. J Wound Care. 2016 Jul 1;25(Sup7):S10-S17. doi: 10.12968/jowc.2016.25.Sup7.S10. PMID: 29027852.