See You at Our Virtual Booth on March 22nd, during WoundCon Spring 2024!
We are pleased to be a part of WoundCon Spring 2024 and are delighted to offer valuable educational opportunities at our virtual booth to the hundreds of dedicated Healthcare Professionals attending.
Join our educational sessions at the times listed below:
This year we are hosting two educational sessions with Lawrence Chen, DPM and Leta McCarty Messinger, BSN, RN, SWOC, who will share their clinical experience with wound healing utilizing Neox®, cryopreserved, ultra-thick human birth tissue allografts as an adjunct to help achieve optimal healing.

Why Neox® Human Birth Tissue Allografts in WoundCare? | 11:40 AM - 12:00 PM ET
Leta McCarty Messinger is the Assistant Director of the Wound Program at Community Hospital of the Monterey Peninsula. In this educational session, Leta will share her objective observations and clinical experience using the Neox family of adjunct therapy wound products to achieve expedited optimal healing outcomes, with fewer applications, across complex and chronic wound applications.

Managing Complex Wounds Utilizing Neox Human Birth Tissue Allografts | 12:00 - 12:20 PM ET
Lawrence Chen, DPM is a multilingual, highly trained, licensed foot and ankle surgeon dedicated to providing and improving patient care through an interdisciplinary team approach. In this session, Dr. Chen will present impactful lower extremity wound healing cases using Neox human birth tissue allografts as an adjunct therapy to achieve optimal outcomes.
Biological Tissue Provides theMost Human Form of Healing
We are in a race against time to heal chronic wounds. BioTissue Surgical products provide the natural healing properties of human birth tissue to the wound.
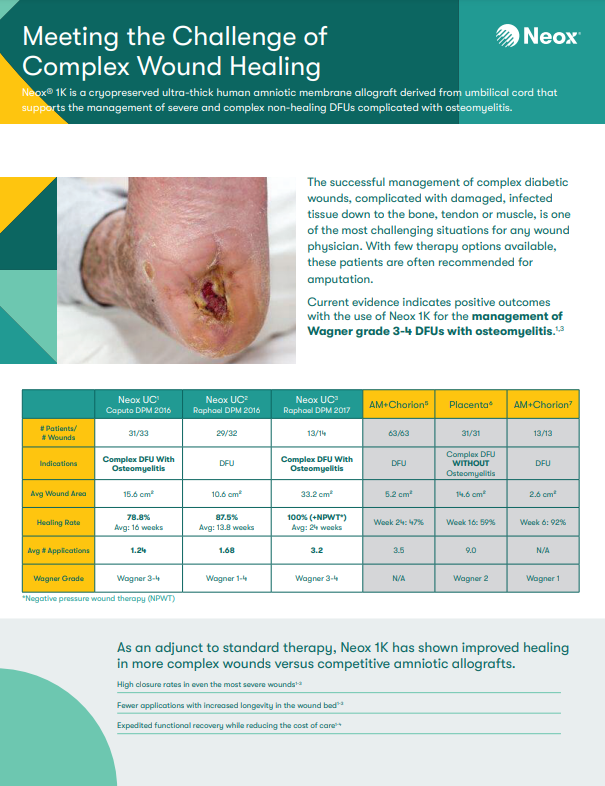
Neox® 1K is a cryopreserved ultra-thick human amniotic membrane derived from umbilical cord used as an adjunct treatment for chronic and acute, partial and full-thickness wounds.1,3-5 This allograft helps promote healing in complex diabetic foot ulcers complicated with osteomyelitis, comparing favorably to the Standard of Care.1,5,6 It’s up to 10 times thicker than amniotic membrane alone,7 which may increase longevity in the wound bed, potentially reducing applications and cost of care.
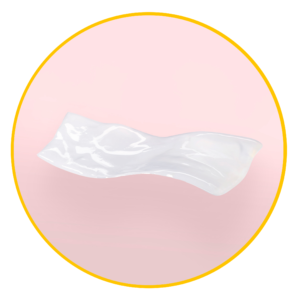
Neox 100 is a thinner cryopreserved version of our human amniotic membrane allograft, ideal for shallow wounds or for larger wound areas. The allograft is delivered on a non-implantable, gridded paper backing for easier handling and application.

Neox RT is a sterile, ultra-thick human amniotic membrane allograft derived from umbilical cord, for chronic and acute partial and full thickness wounds. Neox RT delivers the benefits of human amniotic allograft in a shelf stable product with room temperature storage. It is manufactured, using the SteriTek® preservation process, stored in saline (0.9% w/v NaCl)and terminally sterilized via gamma irradiation yielding a hydrated shelf stable product.
Downloadable Resources