September 14, 2020
Encouraging Outcomes Data with Surgical Repair of Chronic, Large, Refractory Macular Holes Using AmnioGraft® Cryopreserved Amniotic Membrane
Preliminary case series show that AmnioGraft use in the repair of persistent or recurrent Macular Hole defects can lead to improved visual recovery and retinal morphology
Miami, FL – September 14, 2020 — BioTissue, Inc., a TissueTech, Inc. company and leader in the clinical application of Cryopreserved Amniotic Membrane and Umbilical Cord tissue allografts for ocular surface disease, announced today the case series outcome of surgical repair of large macular holes using AmnioGraft Cryopreserved Amniotic Membrane. Results have shown that the placement of AmnioGraft may be a feasible alternative to conventional surgical methods to improve anatomic and visual outcomes.1
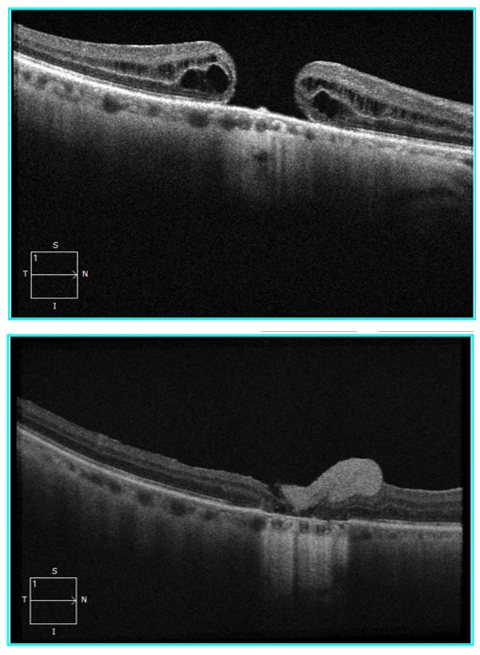
Large, persistent macular holes (MHs) and macular hole retinal detachment can be very challenging to manage. These conditions can cause severe visual impairment, especially in highly myopic eyes.1 – 4 Conventional surgical methods have variable outcomes in terms of closure rates and visual recovery.
AmnioGraft was used in ten patients with significant (1229.30 ± 774.54 μm), persistent or primary MHs in a recent interventional case series from Taiwan.5 After following up with patients for at least six months, eight cases (80%) had sealed MHs, and none of the eyes showed signs of infection, inflammation, or rejection.
Similarly, in another recent review, AmnioGraft improved the best-corrected visual acuity in two cases of chronic, refractory, large MHs. One of the patients was a 65-year old female with a 1,310 μm diameter MH of three years’ duration that had previously failed repair. After repair with AmnioGraft, the patient’s visual acuity improved from 20/100 to 20/60 with the closure of the MH at two weeks and an improving retinal morphology at ten weeks.
“The surgical techniques and the postoperative visual acuity outcomes are very encouraging,” said Ajay Kuriyan, MD, MS. Dr. Kuriyan is the Assistant Professor of Ophthalmology at Sidney Kimmel Medical College, Thomas Jefferson University, Mid Atlantic Retina, and The Retina Service, Wills Eye Hospital. “This use of AmnioGraft is a promising new option for managing chronic, large, refractory macular holes.”
AmnioGraft is cryopreserved using proprietary CRYOTEK® technology to orchestrate an environment that promotes regenerative healing. The CRYOTEK process is the only method proven to effectively retain the Heavy Chain Hyaluronic Acid/Pentraxin3 (HC-HA/PTX3) complex to preserve and deliver the anti-inflammatory and anti-scarring properties of that matrix.
Cryopreserved Amniotic Membrane (AM) and Umbilical Cord (UC) allografts are also the only available products that are shown to be equivalent to fresh AM/UC and superior to dehydrated allografts. In a study published in the Journal of Wound Care, investigators found that cryopreservation retains the native architecture of the AM/UC extracellular matrix and maintains the quantity and activity of key biological signals present in fresh AM/UC. These include high molecular weight hyaluronic acid, heavy chain-HA complex, and pentraxin three. In contrast, dehydrated tissues almost completely lacked these crucial components and were structurally compromised.5 In another study published in the Journal of Investigative Ophthalmology & Visual Science, the cryopreservation process better preserves the structural integrity and biochemistry of AM tissue grafts in comparison to dehydrated grafts and suggests the therapeutic benefit of dehydrated AM may be compromised as a result.6
About BioTissue, Inc.
BioTissue, Inc., Inc., a TissueTech, Inc. company, is the market leader in the clinical application of Cryopreserved Amniotic Membrane-based products for the treatment of diseases and disorders of the ocular surface. Established in 1997, BioTissue serves an unmet need for better surgical and therapeutic alternatives for helping eye care professionals manage ocular surface conditions, such as keratitis, recurrent corneal erosions, conjunctivochalasis, pterygium, and dry eye. Connect with BioTissue on our Physician Portal (for healthcare professionals only), Website, Facebook, LinkedIn, and Twitter.
About TissueTech, Inc.
TissueTech, Inc., the parent company of BioTissue, Inc. and Amniox Medical, Inc., pioneered the development and clinical application of human birth tissue-based products. Founded in 1997, BioTissue markets products for the ophthalmology and optometry markets; and Amniox markets products for use in the musculoskeletal and wound care markets. Clinicians have performed more than 500,000 human implants with the Company’s products and published more than 360 peer-reviewed studies supporting its products and platform technology. The Company’s first product, AmnioGraft®, is the only tissue graft designated by the FDA to act as an anti-inflammatory anti-scarring and anti-angiogenic agent. Learn more at https://biotissue.com/.
