May 3, 2023
Seven-Year Study Shows Improved Clinical Outcomes Following Pterygium Surgery Using TissueTuck Technique with BioTissue AmnioGraft
Data Indicates Sutureless Surgical Method Saves Time, Reduces Recurrence and Complications
MIAMI, May 1, 2023—Recently published findings of a seven-year study indicate that pterygium excision surgery using the TissueTuck sutureless technique with BioTissue AmnioGraft® cryopreserved amniotic membrane results in minimal surgery time and low recurrence and complication rates. A subset of this research, conducted by Neel R. Desai, M.D., will be shared in a paper presentation at the 2023 ASCRS Annual Meeting later this week.
Dr. Desai is among the nation’s foremost experts in ocular surface disease. The study is one of the largest pterygium studies to date, including the analysis of 582 eyes of 453 patients who presented with pterygium between January 2012 and May 2019.
Pterygium is a common ocular surface disease characterized by abnormal fibrovascular tissue of the conjunctiva that encroaches onto the cornea and causes visual impairment. After pterygium excision, a gap is created between the remaining conjunctiva and Tenon capsule. TissueTuck technique uses cautery to seal the gap, followed by the placement of cryopreserved amniotic membrane—BioTissue AmnioGraft—that is tucked into the gap to prevent recurrence and improve cosmesis without complications.
“Aside from sealing the gap, this surgical technique offers several other advantages. The operative procedure takes less than 15 minutes on average, which is significantly shorter than the 30 to 60 minutes in procedures that use conjunctival autografts,” said Dr. Desai. “This surgery also preserves the conjunctiva in case of future glaucoma surgery and has innate biological properties known to reduce inflammation, scarring, and angiogenesis.”
The study found that the average time of TissueTuck pterygium excision surgery was 14.7 minutes. Recurrence rate was 2.3% but only 0.7% in cases with primary, single-headed pterygium without mitomycin C treatment (MMC). The BioTissue AmnioGraft amniotic membrane tissue remained secured to the ocular surface throughout the postoperative period.
“At BioTissue, everything we do to discover, develop, and bring to market products and applications is centered on helping physicians as healers and improving the lives of patients. The overwhelmingly positive results of this study are evidence of progress toward that goal,” said Ted Davis, Chief Executive Officer of BioTissue. “It’s exciting to see physicians establish innovative surgical techniques utilizing our groundbreaking products, and with seven years of data showing the efficacy of the TissueTuck technique, we hope to see it become a standard of care.”
Dr. Desai will present his related paper, Outcomes of the TissueTuck Surgical Technique for Recurrent Pterygium, at ASCRS 2023, on Saturday, May 6 at 1:40 p.m. in Room 1B at the San Diego Convention Center.
Dr. Desai and Bryan Adams, MS3, first shared results of their study last fall in Cornea: The Journal of Cornea and External Disease. The report can be found via open access at https://pubmed.ncbi.nlm.nih.gov/36130320/ Dr. Desai practices ophthalmology at the Eye Institute of West Florida and is a BioTissue speaker, consultant, and stockholder.
About BioTissue Holdings Inc.
BioTissue is an emerging biotechnology company and leader in harnessing the unique power of human birth tissue to support regenerative healing for ocular surface disease and in surgical, chronic wound, and musculoskeletal applications. BioTissue’s portfolio of cryopreserved amniotic membrane products use its proprietary CryoTek® cryopreservation technology, designed to retain the tissue’s structural and functional integrity. The company continues to break new ground with multiple investigational new drug clinical trials as the company pursues biologics license applications for products to treat patients’ unmet clinical needs. BioTissue is committed to empowering healthcare professionals with solutions to deliver optimal patient outcomes by fostering innovation through evidence-based science. Since its inception, clinicians have performed over 790,000 human implants with its products and published over 390 peer-reviewed studies supporting BioTissue’s platform technology. Learn more at biotissue.com.
Media Contact
Heather Kowalczyk, APR
McDougall Communications for BioTissue
[email protected] or (585) 434-2148
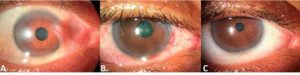
Representative case. Pre-operative pterygium with moderate vascularity and anteriorly displaced semilunar fold with poor anatomic definition (A). Postoperative day one after TissueTuck technique with AMT, without MMC (B). Postoperative day 21 shows excellent cosmesis and restored anatomic integrity of the reconstructed semilunar fold (C).

Neel R. Desai, MD
